Homogeneous catalysis
In chemistry, homogeneous catalysis is a sequence of reactions that involve a catalyst in the same phase as the reactants. Most commonly, a homogeneous catalyst is codissolved in a solvent with the reactants.
Contents |
Examples
Acid catalysis
The proton is the most pervasive homogeneous catalyst [1] because water is the most common solvent. Water forms protons by the process of self-ionization of water In an illustrative case, acids accelerate (catalyse) the hydrolysis of esters:
- CH3CO2CH3 + H2O
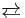 CH3CO2H + CH3OH
CH3CO2H + CH3OH
In the absence of acids, aqueous solutions of most esters do not hydrolyze at practical rates.
Organometallic chemistry
Processes that utilize soluble organometallic compounds as catalysts fall under the category of homogenous catalysis, as opposed to processes that use bulk metal or metal on a solid support which are examples of heterogeneous catalysis. Some well-known examples of homogeneous catalysis include hydroformylation and transfer hydrogenation, as well as certain kinds of Ziegler-Natta polymerization and hydrogenation.[2]
Many non-organometallic complexes are also widely used in catalysis, e.g. for the production of terephthalic acid from xylene.
Other forms of homogeneous catalysis
Enzymes are homogeneous catalysts that are essential for life but are also harnessed for industrial processes. A well studied example carbonic anhydride, which catalyzes the release of CO2 into the lungs from the blood stream.
Contrast with heterogeneous catalysis
Homogeneous catalysis is the opposite of heterogeneous catalysis, where the catalyst is in a different phase than the reactants. One example of heterogeneous catalysis is the petrochemical alkylation process, where the liquid reactants are immiscible with a solution containing the catalyst. Heterogeneous catalysis offers the advantage that products are readily separated from the catalyst, and heterogeneous catalysts are often more stable and degrade much slower than homogeneous catalysts. However, heterogeneous catalysts are difficult to study, so their reaction mechanisms are often unknown.[3]
Enzymes possess properties of both homogeneous and heterogeneous catalysts. As such, they are usually regarded as a third, separate category of catalyst.
References
- ^ R.P. Bell "The Proton in Chemistry", Chapman and Hall, London, 1973. doi: 10.1016/0022-2860(76)80186-X
- ^ Elschenbroich, C. ”Organometallics” (2006) Wiley-VCH: Weinheim. ISBN 978-3-29390-6
- ^ G. O. Spessard and G. L. Miessler "Organometallic Chemistry", Prentice Hall, Upper Saddle River, NJ, 1997, pp. 249-251.